Healgen Rapid Antibody Tests
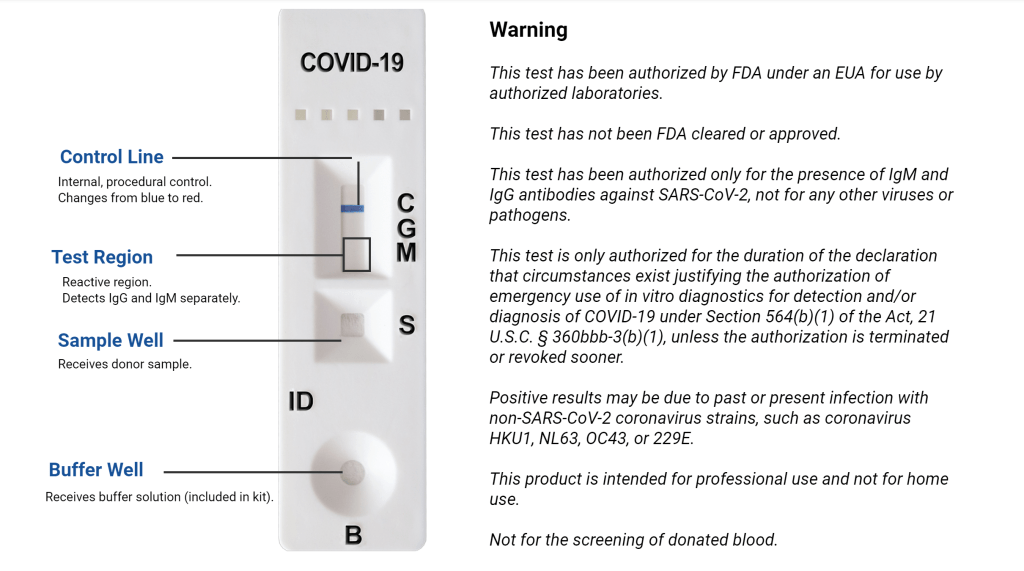
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies. In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for SARS-CoV-2 antibodies.
Healgen Rapid Antibody Test

Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals and birds that cause respiratory, enteric, hepatic and neurologic diseases. Four viruses – 229E, OC43, NL63 and HKU1 are prevalent and typically cause common cold symptoms in immunocompromised individuals. Three other strains SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) can be transmitted from between non-human vertebrates to humans.
COVID-19
Healgen Rapid COVID-19 Antibody Test
A Rapid Point of Care Test
- Detection Window (IgM): 3-5 days after incubation
- Dual band results for simple interpretation
- Multivariable analysis of immunoglobin IgG & IgM
- Room temperature storage or refrigerated (2-30⁰C / 36-86⁰F)
- Procedural internal control included
- Buffer included
COVID Clinical Evaluation
COVID-19 Sensitivity
95.7%
Specificity
100%
Kit Components
- 25 Test devices
- 1 Instruction for Use
FAQ
What is the extraction buffer?
The extraction buffer includes components to dissociate viral proteins from their surfaces to be used as a target for the test. The method of the extraction buffer is used to prepare the test sample by dissolving the virus shell, which also deactivates the virus.
Can I collect the specimen and test at a later time?
The specimen should be tested immediately after collection. The specimen can be retained up to 1 hour following collection if immediate testing of specimens is not possible. Dispose of the specimen and recollect if retained for more than 1 hour.